Transesterification is the process of converting the oil produced from vegetables into biodiesel. The process is less complicated, and it is quite easy. Transesterification is a chemical-based production of biodiesel from Jatropha oil.
In this process, a complex fatty acid like triglyceride molecule is taken and it is neutralized. The glycerin is removed, and an alcohol ester is created. This process is completed when methanol is mixed with sodium hydroxide, which results in the production of sodium methoxide, which is then mixed with oil produced from the Jatropha seeds. When the mixture settles, glycerin is left at the bottom, and the biodiesel (methyl esters) remains on the top. This methyl ester is washed and then filtered.
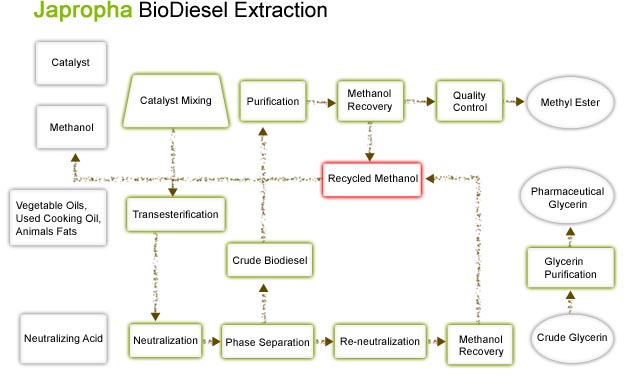
For a more precise understanding of biodiesel extraction from the Jatropha seeds see the Biodiesel Extraction chart
Transesterification Biodiesel Process
Raw materials required
- Jatropha oil
- Methanol
- Potassium hydroxide
- Isopropyl alcohol
- Distilled water
- Phenolphthalein solution
- Vinegar
- Water
Manufacturing Process
- First, Jatropha oil is filtered to remove the solid particles present in it. Then it is heated to remove the water contents if present in it.
- Biodiesel is now used as a diesel engine in the number of agencies. The results are said to be comparable with petroleum in all areas like power, efficiency, climbing, hauling, etc.
- To determine the amount of catalyst that is required.
- The right amount of potassium hydroxide is mixed with methanol until the methanol completely dissolves to get potassium methoxide.
- In the winter season, additional Jatropha oil is heated and is mixed with the potassium methoxide.
- The mixture is allowed to settle. In this process, glycerin settles at the bottom and the biodiesel at the top. The glycerin is then removed, and the biodiesel is washed and dried.
- The biodiesel obtained is then checked for quality.
Note:
In this process, when methanol and potassium hydroxide produces potassium methoxide, which, when mixed with oil, creates a strong polar bond. This breaks the fatty acids into glycerin and biodiesel (esters). These esters later become methyl esters. When the KOH is treated with ethanol, it will form ethyl esters.
(edited 2-Dec-2019)
